Discovery Could Ignite "Engine Of The Future"
 Researchers at Tufts University have
discovered that it's possible to make hydrogen from fossil fuels
using far less platinum or gold than current fuel processing
technology has required. Their research shows that 90 percent of
precious metals used today may be removed from the catalyst without
affecting its ability to produce hydrogen.
Researchers at Tufts University have
discovered that it's possible to make hydrogen from fossil fuels
using far less platinum or gold than current fuel processing
technology has required. Their research shows that 90 percent of
precious metals used today may be removed from the catalyst without
affecting its ability to produce hydrogen.
This finding could have potential cost savings of millions of
dollars in the materials required to commercialize the fuel cell
technology.
The research will be published in the July 3 edition of
"Science Express," the online version of the journal
Science that provides rapid electronic publication of
timely and important research papers. The article also will be
published in Science later this summer.
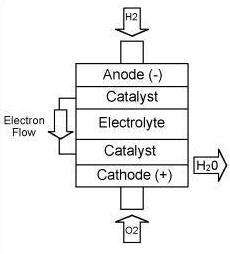 A fuel cell consists of two
electrodes sandwiched around an electrolyte. Hydrogen fed to the
one electrode (anode) passes through the electrolyte in the form of
protons and combines with oxygen on the other electrode (cathode)
making water and producing heat. Electricity is generated in the
process. A fuel cell will produce energy in the form of electricity
and heat as long as fuel and oxygen are supplied. To produce
fuel-cell quality hydrogen, an important step involves the removal
of any by-product carbon monoxide, which poisons the fuel cell
anode catalyst.
A fuel cell consists of two
electrodes sandwiched around an electrolyte. Hydrogen fed to the
one electrode (anode) passes through the electrolyte in the form of
protons and combines with oxygen on the other electrode (cathode)
making water and producing heat. Electricity is generated in the
process. A fuel cell will produce energy in the form of electricity
and heat as long as fuel and oxygen are supplied. To produce
fuel-cell quality hydrogen, an important step involves the removal
of any by-product carbon monoxide, which poisons the fuel cell
anode catalyst.
"A lot of people have spent a lot of time studying the
properties of gold and platinum nanoparticles that are used to
catalyze the reaction of carbon monoxide with water to make
hydrogen and carbon dioxide," said Maria Flytzani-Stephanopoulos,
professor of chemical and biological engineering at Tufts and the
lead researcher of the project. "We find that for this reaction
over a cerium oxide catalyst carrying the gold or platinum, metal
nanoparticles are not important. Only a tiny amount of the precious
metal in non metallic form is needed to create the active catalyst.
Our finding will help researchers find a cost-effective way to
produce clean energy from fuel cells in the near future"
She and her two colleagues, doctoral student Qi Fu and research
professor Howard Saltsburg, were funded by a $300,000 three-year
grant from the National Science Foundation, and have filed a
provisional patent for their research. Their cutting-edge work in
catalytic fuel processing to generate hydrogen for fuel cell
applications is one of the major undertakings at Tufts' Science and
Technology Center at the University's Medford campus (MA).
The Tufts researchers' article is based on the "water-gas shift"
reaction they use to make hydrogen from water and carbon monoxide
over cerium oxide loaded with gold or platinum. Typically, a
loading of 1-10 weight percent of gold or other precious metals is
used to make an effective catalyst. But the Tufts team discovered
that, after stripping the gold with a cyanide solution, the
catalyst was just as active with a slight amount of the gold
remaining – one-tenth the normal amount used.
According to Flytzani-Stephanopoulos, "This finding is
significant because it shows that metallic nanoparticles are mere
'spectator species' for some reactions, such as the water-gas
shift. The phenomenon may be more general, since we show that it
also holds for platinum and may also hold true for other metals and
metal oxide supports, such as titanium and iron oxide."
She adds, "It opens the way for new catalyst designs so more
hydrogen can be produced with less precious metal. This can pave
the way for cost-effective clean energy production from fuel cells
in the near future."
Fuel cells currently are being used on a trial basis in some
buses, cars and even hotels, but they're expensive. It may take up
to 10 years until the technology is used in transportation and by
the general population. (Since the 1960s, one type of fuel cell has
powered NASA's spacecrafts.)
"We've raised the issue of now having to look back and see if
less precious metal may be used in other similar applications,"
said Saltsburg. There's much more to be done, and that's what makes
the research exciting."
 ANN's Daily Aero-Term (12.01.25): Convective SIGMET
ANN's Daily Aero-Term (12.01.25): Convective SIGMET ANN's Daily Aero-Linx (12.01.25)
ANN's Daily Aero-Linx (12.01.25) NTSB Final Report: Remos Aircraft GmbH Remos GX
NTSB Final Report: Remos Aircraft GmbH Remos GX Aero-News: Quote of the Day (12.02.25)
Aero-News: Quote of the Day (12.02.25) ANN's Daily Aero-Term (12.02.25): Coupled Approach
ANN's Daily Aero-Term (12.02.25): Coupled Approach





